Abstract
B-cell lymphoma 2 (BCL2) family proteins play an important role in the intrinsic mitochondrial apoptotic response. As such, selective small-molecule BCL2 inhibitor venetoclax has proven to be an effective therapeutic in hematologic malignancies including acute myeloid leukemia (AML). The biggest pitfall of venetoclax, however, is that here is no clear biomarker that can readily predict its response. Discovery of molecular markers for response prediction based on genomics, BCL2 expression level, and anti-apoptotic protein level has not been successful so far. We hypothesized that venetoclax was essentially developed as a protein-protein interaction (PPI) drug, and since PPI is complexly involved in metabolic processes including post-translational modification of the corresponding protein, it is difficult to evaluate its level with genetic mutation or protein expression level. Therefore, in order to evaluate a PPI drug such as venetoclax, a study through direct PPI observation should be preceded, and changes in the PPI pathway that the PPI drug targets should be confirmed. Using single molecule co-immunoprecipitation technology, we sought to predict the status of each anti-apoptotic protein and identify the BCL2 related PPI, which is the main target of venetoclax.
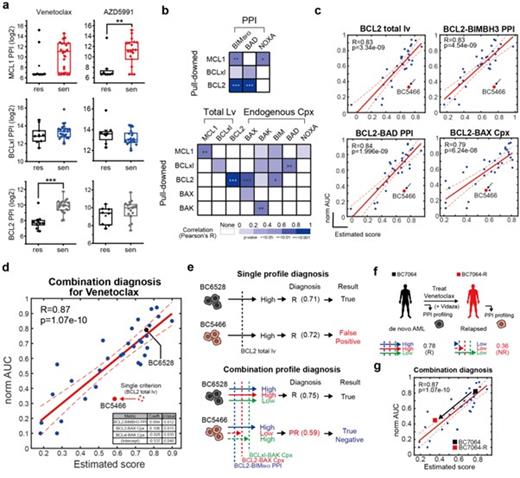
First, we tried to check whether the size of the PPI portion measured using the PPI probe is related to the reactivity of the BH3 mimetics of each sample. Based on the EC50 data obtained through treatment with venetoclax and AZD5991, the sample was divided into a sensitive group (EC50<300nM) and a resistance group with low reactivity (EC50>300nM), which were highly responsive to BH3 mimetics, and the difference in PPI between the two groups was analyzed. (Figure A) As a result of the analysis, it was confirmed that the BCL2 related profile, especially the BCL2 total lv and the BCL2 PPI, had a strong positive correlation with the venentoclax norm-AUC as initially predicted. (Figure B) Also, the level of the BCL2-BAX complex had a positive correlation with the venetoclax sensitivity, and these four types of profiles had a significantly higher correlation than the others. (Figure C) In the estimation process, models that do not fit known biology were excluded, and models with a low significance level were also excluded. As a result of estimation, it was confirmed that the model with the strongest predictive power was formed when 1) BCL2-BIMBH3 PPI, 2) BCL2-BAX complex, and 3) BCLxl-BAK complex were combined (Figure D, E). The accuracy of the model was r=0.87. The validation of the combination diagnosis model proceeded by tracking the PPI change of the primary bone marrow sample of patients who received venetoclax in clinic. Patient number BC7064 was classified as a high-BCL2 "responsive" group and was treated with venetoclax plus azacitidine. After stopping drug treatment, AML relapsed (BC7064-R) (Figure F). We conducted combination diagnosis on BC7064 and BC7064-R samples, and confirmed that the BCL2-BIMBH3 PPI decreased by half after relapse. In particular, the BCL2-BAX complex was no longer detected after relapse, and thus it could be predicted that BC7064-R will have significantly lower venetoclax responsiveness (Figure G).
In conclusion, the combination diagnosis based on the BCL2 profile proposed by this research was made based on in vitro drug sensitivity, but it could also be applied to the actual clinical response results. In addition, this diagnostic method can sensitively track changes in the BCL2 profile throughout the AML treatment course, as seen in BC7064 case.
Koh: Pfizer: Consultancy; Jassen: Honoraria; AstraZeneca: Honoraria; Novartis: Honoraria; GSK: Honoraria; Roche: Honoraria; Takeda: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal